Proton Exchange Membrane (PEM) Fuel Cell: What is it, and how does it work?
The proton exchange membrane (PEM) fuel cell is a progressive technology that produces clean electricity by converting hydrogen and oxygen into electric energy. The PEM-fuel cell consists of many cells separated by a conductive membrane. Hydrogen is supplied on the anode side, while oxygen or air is provided on the cathode side. The chemical reaction between hydrogen and oxygen in the counter ward of the membrane causes the production of electric energy and water as the only side product.
Why are the PEM Fuel Cell and Hydrogen currently so urgently needed?
Both PEM-fuel cells and hydrogen play a vital role in shaping a sustainable and environmentally friendly energy future. With longer operating ranges and faster refueling, the PEM-fuel cells became a necessary alternative to battery-powered motor vehicles. Furthermore, they serve as a decentral power supply in your home, especially in combination with solar cells, to save green energy and only use it when necessary. The wide application of hydrogen as a clean energy source can help reduce CO₂ emissions and slow down climate change.
Proton Exchange Membrane (PEM) Electrolyzer: Deployment and Utilization
The proton exchange membrane electrolyzer is a crucial element in hydrogen production and plays an essential role in using renewable energies. The electrolyzer uses the PEM-fuel cell technology inversely by obtaining hydrogen and oxygen from water with electrical energy. Through the application of electrical voltage to the PEM-membrane, water is split into its original parts – hydrogen and oxygen. The extracted hydrogen can now be stored as green energy and later used, for example, to power vehicles, heat buildings, or supply industrial processes. PEM-electrolyzers are effective environmental instruments for producing green hydrogen and are crucial in shaping a sustainable hydrogen economy.
New National Hydrogen Strategy of the Federal Government of July 27, 2023
The federal government has noticed hydrogen’s massive potential for the Energiewende, pursuing a new ambitious hydrogen strategy. Until 2023 the generation capabilities are supposed to be doubled to 10 gigawatts, which could cover 20-30 percent of Germany’s hydrogen demand. This strategy would need expanding plants, storage facilities, and services. A particular focus is on increasingly producing hydrogen from renewable energies to achieve sustainability targets. The vision is to make Germany climate-neutral by 2045.
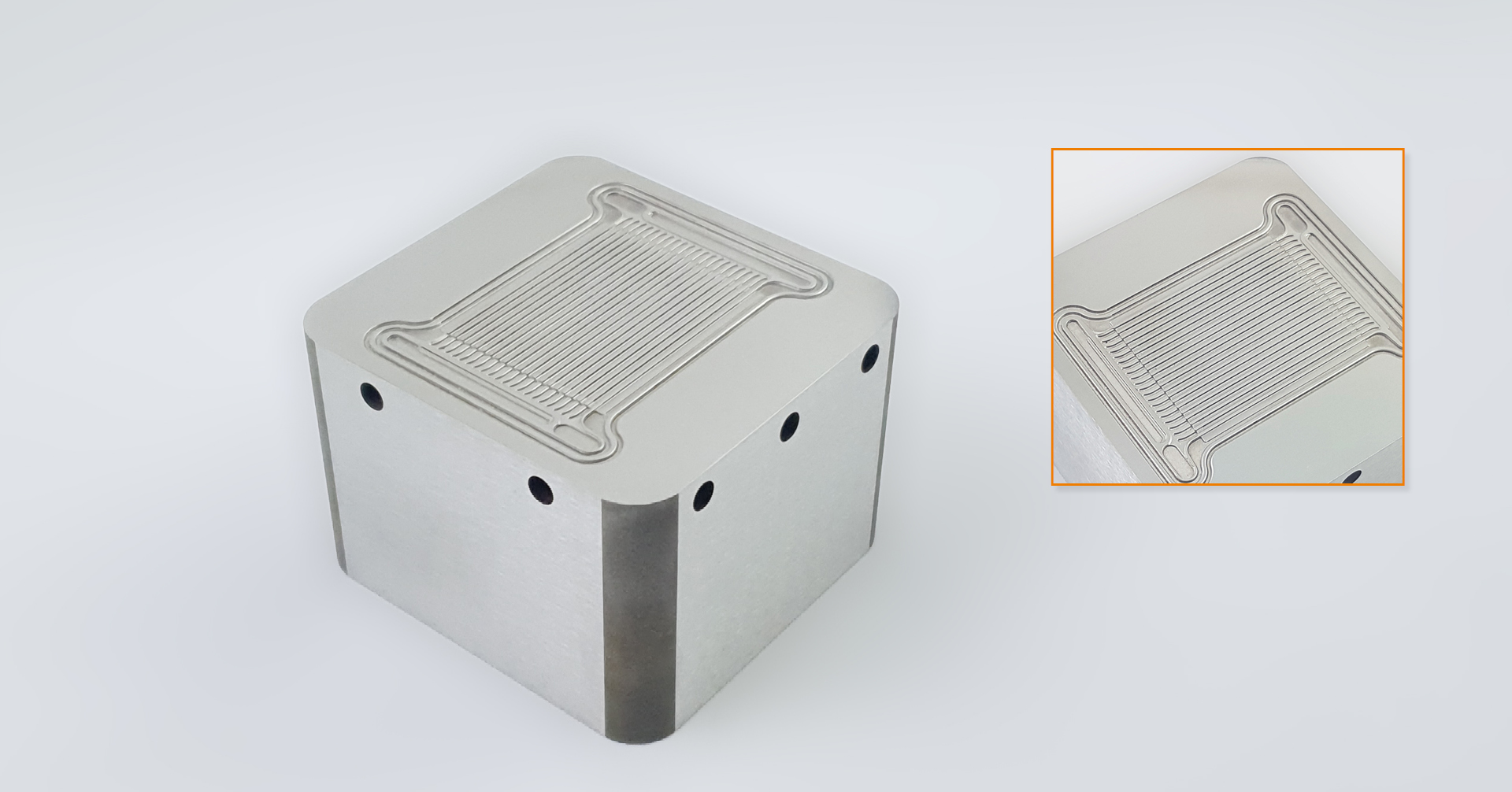
Components of the PEM Fuel Cell (PEMFC stack)
In the bipolar stack design, the individual cells are electrically contacted via a common bipolar plate. Through the bipolar plate on one side, the fuel gas leads to the respective electrodes, and on the other side, air or oxygen leads there. Its name does the bipolar plate get of the voltage applied on both sides: minus on the anode side (hydrogen), plus on the cathode side (oxygen). The stack voltage describes the sum of the single-cell voltages, which are dissipated via current collectors or pantographs at the beginning and end of the cell stack. The stack voltage depends on the size of the active space meaning that doubling the space also doubles the electricity.
The PEM fuel cell consists of multiple layers and components arranged in a stack and works together to make energy conversion possible. Here are the essential components: